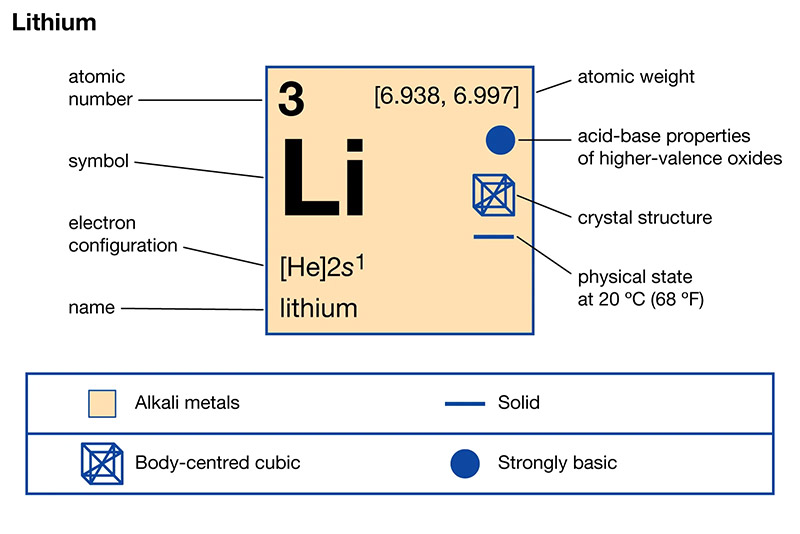
Last Updated on July 23, 2023
Short Answer
The element that has similar properties to lithium is sodium. Both lithium and sodium belong to the alkali metal group and have similar chemical behaviors. They both react vigorously with water, form ionic compounds, and have low melting and boiling points. Sodium, like lithium, is also highly reactive and can be found in various compounds and minerals. However, it is important to note that while sodium shares some similarities with lithium, there are also distinct differences in their properties and reactivity.
Welcome to this exploration of the similar properties of the element lithium. In the world of chemistry, understanding the importance of similar properties is crucial. By examining the alkali metals group in the periodic table, we can begin to unveil the similarities between lithium and other elements. In this article, we will delve into the similarities between lithium and sodium, potassium, rubidium, cesium, and even francium. It is truly fascinating to discover the commonalities that exist among these elements. So, let’s embark on this journey of exploration and unravel the intriguing similarities of the lithium element and its alkali metal counterparts.
Understanding the Importance of Similar Properties in Chemistry
In the field of chemistry, understanding the similar properties of elements is of utmost importance. These similarities allow scientists to make predictions about the behavior and reactions of different elements. When two elements have similar properties, it means that they have similar chemical behaviors and can often be used interchangeably in various chemical reactions.
Here are some key points to consider:
- Similar properties of elements help in categorizing them into groups in the periodic table.
- Elements with similar properties often have similar electron configurations.
- Similar properties allow scientists to make predictions about the reactivity and behavior of elements.
- Knowing the similar properties of elements can help in the development of new materials and compounds.
Understanding the importance of similar properties in chemistry is crucial for further advancements in the field and for expanding our knowledge of the elements.
Exploring the Alkali Metals Group in the Periodic Table
The alkali metals group is a fascinating group of elements located in the periodic table. These elements include lithium, sodium, potassium, rubidium, cesium, and francium. They are known for their similar properties and characteristics, which make them stand out from other elements.
One important characteristic of alkali metals is their low density. This means that they are lighter than most other elements, making them easy to handle and transport. Another key property is their high reactivity. Alkali metals are highly reactive and can easily react with other elements or compounds.
Alkali metals also have a single valence electron. This electron is located in the outermost shell of the atom and is responsible for the element’s chemical behavior. It is this single valence electron that gives alkali metals their unique properties.
Furthermore, alkali metals are excellent conductors of heat and electricity. This makes them useful in various applications, such as in batteries and electrical circuits. They also have low melting and boiling points, which means they can easily change from solid to liquid or gas state.
In conclusion, the alkali metals group in the periodic table is a fascinating group of elements with similar properties. Their low density, high reactivity, single valence electron, and excellent conductivity make them unique and valuable in various industries. Understanding the similarities between these elements, such as lithium, sodium, potassium, rubidium, cesium, and francium, can help us further explore their potential applications and contributions to the field of chemistry.
Unveiling the Similar Properties of Lithium and Sodium
When it comes to exploring the similar properties of elements, one cannot overlook the fascinating similarities between lithium and sodium. Both of these elements belong to the alkali metals group in the periodic table, which is known for its highly reactive nature.
One of the key similarities between lithium and sodium is their ability to readily lose an electron, making them highly reactive. This property is due to their outermost electron configuration, which consists of only one electron. As a result, both lithium and sodium exhibit similar chemical behaviors, such as forming compounds with other elements.
Another similarity between lithium and sodium is their low melting and boiling points. This characteristic is a result of the weak metallic bonding between their atoms, allowing them to easily transition from solid to liquid states at relatively low temperatures.
Furthermore, both lithium and sodium are highly reactive with water, producing hydrogen gas and hydroxide ions. This reaction is exothermic, releasing a significant amount of heat. It is important to handle these elements with caution due to their reactivity.
In conclusion, the similarities between lithium and sodium are truly fascinating. From their ability to lose electrons to their low melting and boiling points, these elements share several common properties. Understanding these similarities is crucial in the field of chemistry as it helps us predict the behavior of elements and their compounds.
Examining the Similarities Between Lithium and Potassium
When it comes to exploring the similar properties of the element lithium, it is important to also consider its counterparts in the alkali metals group. One such counterpart is potassium, which shares several similarities with lithium:
- Atomic Structure: Both lithium and potassium have similar atomic structures, with lithium having an atomic number of 3 and potassium having an atomic number of 19.
- Chemical Reactivity: Like lithium, potassium is highly reactive and can easily form compounds with other elements.
- Electronegativity: Both lithium and potassium have low electronegativity values, meaning they have a tendency to lose electrons rather than gain them.
- Physical Properties: Both elements have low melting and boiling points, making them relatively easy to melt and vaporize.
- Similar Uses: Lithium and potassium are both used in various applications, such as in batteries, alloys, and pharmaceuticals.
By examining the similarities between lithium and potassium, we can gain a better understanding of the properties and behaviors of these elements within the alkali metals group.
Discovering the Commonalities of Lithium and Rubidium
When it comes to exploring the similar properties of the element lithium, it is important to also consider its relationship with other elements in the alkali metals group. One such element that shares commonalities with lithium is rubidium. Let’s delve into the fascinating similarities between these two elements:
- Atomic Structure: Both lithium and rubidium belong to the same group in the periodic table, which means they have similar atomic structures. They both have a single valence electron in their outermost energy level.
- Reactivity: Like lithium, rubidium is highly reactive. Both elements readily lose their valence electron to form positive ions.
- Physical Properties: Lithium and rubidium share some physical properties. They are both soft metals that can be easily cut with a knife. Additionally, they have low melting and boiling points.
- Chemical Properties: Both elements exhibit similar chemical properties. They react vigorously with water, producing hydrogen gas and hydroxide ions.
- Uses: Lithium and rubidium find applications in various fields. Lithium is commonly used in batteries, while rubidium is used in atomic clocks and as a catalyst in certain chemical reactions.
By understanding the commonalities between lithium and rubidium, we can gain a deeper insight into the properties and behavior of these elements. This knowledge is crucial in the field of chemistry and helps scientists make advancements in various industries.
Exploring the Similar Properties of Lithium and Cesium
When it comes to exploring the similar properties of the element lithium, one cannot overlook its striking resemblance to cesium. Both lithium and cesium belong to the alkali metals group in the periodic table, which is known for its highly reactive nature.
One of the key similarities between lithium and cesium is their low melting and boiling points. Both elements have relatively low melting points, making them easily transform from solid to liquid states. This property is crucial in various industrial applications, such as the production of batteries and alloys.
Furthermore, lithium and cesium share similar chemical reactivity. They both readily react with water, releasing hydrogen gas and forming hydroxides. This reactivity is due to their outer electron configuration, which makes them highly prone to losing electrons and forming positive ions.
Another intriguing similarity between lithium and cesium is their ability to exhibit explosive reactions with certain compounds. This property is particularly evident when they react with halogens, such as chlorine or fluorine. The resulting compounds can be highly volatile and pose significant safety risks.
In conclusion, the exploration of the similar properties of lithium and cesium sheds light on the fascinating characteristics of alkali metals. Their shared reactivity, low melting points, and explosive tendencies make them essential elements in various scientific and industrial fields.
Unraveling the Similarities Between Lithium and Francium
As we continue our exploration of the similar properties of the element lithium, we now turn our attention to its counterpart in the alkali metals group, francium. Francium, like lithium, is a highly reactive metal that belongs to the same group in the periodic table. Both elements have a single valence electron, which makes them highly reactive and prone to forming compounds.
One of the key similarities between lithium and francium is their ability to readily lose their valence electron, resulting in the formation of a positive ion. This property is what gives them their high reactivity and makes them useful in various chemical reactions.
Additionally, both lithium and francium have low melting and boiling points, which means they can easily change from a solid to a liquid state at relatively low temperatures. This property makes them useful in certain applications, such as in the production of batteries and as catalysts in chemical reactions.
In conclusion, the similarities between lithium and francium highlight the commonalities within the alkali metals group. Their shared properties of high reactivity and low melting points make them important elements in various chemical processes.
Exploring the Similar Properties of Lithium Element and Its Alkali Metal Counterparts
Throughout this article, we have delved into the fascinating world of chemistry to uncover the similar properties shared by the element lithium and its alkali metal counterparts. From sodium to francium, these elements exhibit a range of characteristics that make them stand out in the periodic table.
One of the key aspects we have discovered is the importance of similar properties in chemistry. By understanding the similarities between elements, scientists can make predictions about their behavior and reactions. This knowledge is crucial in various fields, including medicine, energy production, and materials science.
As we explored the alkali metals group, we found that lithium shares similar properties with each of its neighboring elements. From sodium’s reactivity to potassium’s ability to ignite in water, these elements showcase a range of intriguing behaviors.
In conclusion, the fascinating similarities between lithium and its alkali metal counterparts highlight the interconnectedness of the elements in the periodic table. By studying these similarities, scientists can unlock new insights and applications for these elements, paving the way for advancements in various scientific disciplines.
Frequently Asked Questions
Q: What are similar properties in chemistry?
Similar properties in chemistry refer to the characteristics and behaviors that elements or compounds share due to their similar atomic structures or chemical compositions.
Q: What is the Alkali Metals Group in the Periodic Table?
The Alkali Metals Group is a group of elements located in the first column of the periodic table, consisting of lithium, sodium, potassium, rubidium, cesium, and francium. These elements are highly reactive and possess similar chemical properties.
Q: What are the similarities between lithium and sodium?
Lithium and sodium share several similarities. Both elements belong to the Alkali Metals Group, have low melting and boiling points, and are highly reactive. They also exhibit similar chemical reactions and can form compounds with similar properties.
Q: How are lithium and potassium similar?
Lithium and potassium share similarities as they both belong to the Alkali Metals Group. They have similar atomic structures, low densities, and are highly reactive. Additionally, they can form compounds with similar properties.
Q: What are the commonalities between lithium and rubidium?
Lithium and rubidium have commonalities as they are both members of the Alkali Metals Group. They possess similar chemical properties, including high reactivity and the ability to form compounds with similar characteristics. They also have low melting and boiling points.
About The Author

Wendy Lee is a pop culture ninja who knows all the latest trends and gossip. She's also an animal lover, and will be friends with any creature that crosses her path. Wendy is an expert writer and can tackle any subject with ease. But most of all, she loves to travel - and she's not afraid to evangelize about it to anyone who'll listen! Wendy enjoys all kinds of Asian food and cultures, and she considers herself a bit of a ninja when it comes to eating spicy foods.