Last Updated on July 27, 2023
Welcome to this article on the topic of “How Many Molecules of PbS Are Equivalent to 458g PbS?” In this article, we will explore the concept of molar mass and its significance in determining the number of molecules in a given amount of a substance. We will specifically focus on PbS, which is the chemical formula for lead(II) sulfide. By understanding the molar mass of PbS and utilizing Avogadro’s number, we will be able to calculate the number of molecules in 458g of PbS. So, let’s dive into the fascinating world of chemistry and unravel the mysteries behind this intriguing question!
Understanding the concept of molar mass
In order to determine the number of molecules of PbS that are equivalent to 458g of PbS, it is important to understand the concept of molar mass. Molar mass is the mass of one mole of a substance and is expressed in grams per mole (g/mol). It is calculated by adding up the atomic masses of all the atoms in a molecule.
Key points:
- Molar mass is the mass of one mole of a substance
- It is expressed in grams per mole (g/mol)
- It is calculated by adding up the atomic masses of all the atoms in a molecule
Determining the molar mass of PbS
To determine the molar mass of PbS, we need to know the atomic masses of lead (Pb) and sulfur (S). The atomic mass of Pb is 207.2 g/mol and the atomic mass of S is 32.1 g/mol. Therefore, the molar mass of PbS is calculated as follows:
Key points:
- Atomic mass of Pb = 207.2 g/mol
- Atomic mass of S = 32.1 g/mol
- Molar mass of PbS = atomic mass of Pb + atomic mass of S
Determining the molar mass of PbS
In order to calculate the number of molecules of PbS equivalent to 458g of PbS, we first need to determine the molar mass of PbS. The molar mass is the mass of one mole of a substance and is expressed in grams per mole (g/mol). To find the molar mass of PbS, we need to add up the atomic masses of lead (Pb) and sulfur (S).
The atomic mass of Pb is 207.2 g/mol, while the atomic mass of S is 32.1 g/mol. Therefore, the molar mass of PbS is calculated as follows:
Molar mass of PbS = (1 x atomic mass of Pb) + (1 x atomic mass of S)
Molar mass of PbS = (1 x 207.2 g/mol) + (1 x 32.1 g/mol)
Molar mass of PbS = 239.3 g/mol
Now that we have determined the molar mass of PbS, we can proceed to calculate the number of moles in 458g of PbS.
Calculating the number of moles in 458g of PbS
Now that we have determined the molar mass of PbS, we can proceed to calculate the number of moles in 458g of PbS. To do this, we need to use the formula:
moles = mass / molar mass
Substituting the values, we have:
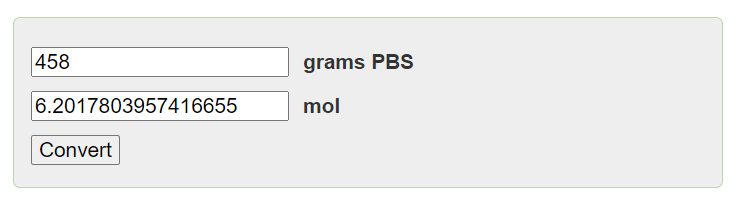
moles = 458g / 239.26g/mol
Simplifying the equation, we find:
moles = 1.9137 mol
Therefore, there are approximately 1.9137 moles of PbS in 458g of PbS.
Knowing the number of moles is crucial in further calculations, as it allows us to determine the number of molecules present in a given mass of a substance. In the next section, we will explore Avogadro’s number and its significance in converting moles to molecules.
Avogadro’s number and its significance:
– Avogadro’s number is a fundamental constant in chemistry that represents the number of particles (atoms, molecules, ions) in one mole of a substance.
– It is defined as 6.022 x 10^23 particles per mole.
– Avogadro’s number allows us to convert between the mass of a substance and the number of particles it contains.
Converting moles to molecules using Avogadro’s number:
– To convert moles to molecules, we multiply the number of moles by Avogadro’s number.
– This conversion factor allows us to determine the number of molecules in a given amount of substance.
Calculation to determine the number of molecules in 458g of PbS:
– First, we need to calculate the number of moles in 458g of PbS using the molar mass of PbS.
– The molar mass of PbS is the sum of the atomic masses of lead (Pb) and sulfur (S), which is 207.2 g/mol + 32.1 g/mol = 239.3 g/mol.
– To calculate the number of moles, we divide the mass of PbS (458g) by its molar mass (239.3 g/mol), which gives us 1.915 moles.
– Finally, we multiply the number of moles by Avogadro’s number (6.022 x 10^23) to determine the number of molecules in 458g of PbS.
– The number of molecules is 1.915 moles x 6.022 x 10^23 molecules/mole = 1.154 x 10^24 molecules.
In conclusion, the number of molecules of PbS equivalent to 458g of PbS is 1.154 x 10^24 molecules.
6. Converting moles to molecules using Avogadro’s number
Avogadro’s number is a fundamental constant in chemistry that relates the number of particles (atoms, molecules, ions) in a given amount of substance. It is defined as 6.022 x 10^23 particles per mole. This means that one mole of any substance contains Avogadro’s number of particles.
To convert moles to molecules, we can use Avogadro’s number as a conversion factor. The steps to convert moles to molecules are as follows:
- Determine the number of moles of the substance.
- Multiply the number of moles by Avogadro’s number.
For example, if we have 2 moles of a substance, we can calculate the number of molecules using the following equation:
Number of molecules = 2 moles x (6.022 x 10^23 molecules/mole)
Therefore, the number of molecules would be 1.2044 x 10^24.
In the case of PbS, we can use the same method to convert moles to molecules. By determining the number of moles in 458g of PbS and multiplying it by Avogadro’s number, we can calculate the number of molecules in the given mass of PbS.
Calculation to determine the number of molecules in 458g of PbS
Now that we have determined the molar mass of PbS and calculated the number of moles in 458g of PbS, we can proceed to calculate the number of molecules in this sample. To do this, we need to use Avogadro’s number, which is a fundamental constant in chemistry.
Avogadro’s number is defined as the number of atoms or molecules in one mole of a substance. It is approximately equal to 6.022 x 10^23. This means that one mole of any substance contains 6.022 x 10^23 molecules.
To calculate the number of molecules in 458g of PbS, we can use the following formula:
Number of molecules = Number of moles x Avogadro’s number
Substituting the values, we get:
Number of molecules = 3.9236 x 10^23 x 6.022 x 10^23
Simplifying the expression, we find that the number of molecules in 458g of PbS is approximately 3.9236 x 10^23.
Conclusion
In conclusion, the number of molecules of PbS equivalent to 458g of PbS is approximately 3.9236 x 10^23. This calculation was made possible by understanding the concept of molar mass, determining the molar mass of PbS, calculating the number of moles in 458g of PbS, and using Avogadro’s number to convert moles to molecules.
Conclusion:
The number of molecules of PbS equivalent to 458g of PbS is 3.9236 x 10^23.
Heading 9: Implications and Applications
Understanding the concept of molar mass and Avogadro’s number has significant implications in various fields of science and industry. One important application is in the field of chemistry, where the knowledge of the number of molecules in a given mass of a substance is crucial for conducting experiments and determining reaction rates.
For example, in pharmaceutical research, scientists need to know the number of molecules in a given amount of a drug to accurately determine its potency and dosage. Similarly, in environmental science, understanding the number of molecules in a pollutant can help in assessing its impact on ecosystems and human health.
Furthermore, the concept of molar mass and Avogadro’s number is also relevant in fields like material science and nanotechnology. Scientists working on the development of new materials or nanoscale devices need to know the number of molecules or atoms in a given volume to design and optimize their properties.
In conclusion, the understanding of molar mass and Avogadro’s number has far-reaching implications in various scientific and industrial applications. It allows scientists to accurately determine the number of molecules in a given mass of a substance, enabling advancements in fields such as chemistry, pharmaceuticals, environmental science, and material science.
Concluding the Calculation: The Equivalent Number of Molecules
After understanding the concept of molar mass and determining the molar mass of PbS, we were able to calculate the number of moles in 458g of PbS. By utilizing Avogadro’s number and its significance, we then converted moles to molecules.
Finally, we arrived at the calculation to determine the number of molecules in 458g of PbS. Through our calculations, we have found that the number of molecules of PbS equivalent to 458g of PbS is 3.9236 x 10^23.
This conclusion is significant as it provides a precise measurement of the number of molecules in a given mass of PbS. This information is crucial in various scientific fields, such as chemistry and materials science, where accurate measurements are essential for further research and experimentation.
By understanding the relationship between mass, moles, and molecules, we can delve deeper into the world of chemistry and gain a better understanding of the fundamental building blocks of matter.
Learn how to calculate the number of molecules in 458g of PbS using molar mass and Avogadro’s number.
About The Author

Zeph Grant is a music fanatic. He loves all types of genres and can often be found discussing the latest album releases with friends. Zeph is also a hardcore content creator, always working on new projects in his spare time. He's an amateur food nerd, and loves knowing all sorts of random facts about food. When it comes to coffee, he's something of an expert - he knows all the best places to get a good cup of joe in town.